What is diazotization? How is benzene diazonium chloride prepared? Describe it's important reactions with mechanism.
The chemical process used in converting a primary aromatic amine into the corresponding diazonium salt of the amine is commonly referred to as diazotization. This process is also known as ‘diazotization’
The German industrial chemist Peter Griess was the first person to report such a reaction in 1858. He went on to discover many more reactions involving diazonium salts.
Generally, the preparation of these diazonium salts involves the reaction of an aromatic amine with nitrous acid in the presence of another acid. An example of a diazotization reaction is given below.
In the example illustrated above, it can be noted that nitrous acid is generated from the reaction between the sodium nitrite and the other mineral acid (acid derived from one or many inorganic compounds) which is present in excess.
The nitrosation of primary aromatic amines with nitrous acid (generated in situ from sodium nitrite and a strong acid, such as hydrochloric acid, sulfuric acid, or HBF4) leads to diazonium salts, which can be isolated if the counterion is non-nucleophilic.
Diazonium salts are important intermediates for the preparation of halides (Sandmeyer Reaction, Schiemann Reaction), and azo compounds. Diazonium salts can react as pseudohalide-type electrophiles, and can therefore be used in specific protocols for the Heck Reaction or Suzuki Coupling.
The intermediates resulting from the diazotization of primary, aliphatic amines are unstable; they are rapidly converted into carbocations after loss of nitrogen, and yield products derived from substitution, elimination or rearrangement processes.
Preparation of benzenediazonium chloride
Chemical properties of benzene diazonium chloride
If temperature is increased in aqueous benzene diazonium chloride, it decomposes to phenol. Therefore, benzene diazonium is prepared when it is required for some purpose.
- Benzene diazonium chloride exists as a colourless solid.
- There is no melting or boiling point values because it decomposes readily.
Join VKSU Help Desk ( On Telegram) – Click Here |
Preparation of benzenediazonium chloride
Benzene diazonium chloride is prepared by aniline. When aniline reacts with nitrous acid under low temperature (0-50C), benzenediazonium chloride is given as the product. If temperature is increased benzene diazonium chloride decomposes to phenol. Therefore, you have to be careful with temperature when you prepare benzene diazonium chloride.
 |
| aniline and nitrous gives benzene diazonium chloride |
Note: In the laboratory, you will not see nitrous acid in your chemical store. Therefore you have to prepare it. Nitrous acid is prepared by mixing aqueous sodium nitrite and cold dilute hydrochloric acid.
Reactions of benzenediazonium chloride
We can categorize reactions of benzenediazonium chloride into two categories.
- Reactions of substituting diazonium group by another group
- Coupling reactions of diazonium ions
Benzene diazonium chloride is used as a raw material in the production of dyes. In next section, we will look those dye preparing reactions too.
Reactions of substituting diazonium group by another group
BenzeneDiazonium chloride (C6H5-N2Cl) can be converted to another important aromatic organic compounds such as chlorobenzene, bromobenzene, phenol and more. -N2Cl group is replaced by another group such as -Cl, -Br, -OH, etc.
Sandmeyer reactions of benzene diazonium chloride
SandMeyer reactions were found in 1884 by a Swiss chemist Traugott Sandmeyer. From sandmeyer reactions, we can prepare chloribenzene, bromebenzene, benzonitrile, iodobenzene and flurobenzene as products.
- Chlorobenzene is formed when benzenediazonium chloride is treated with CuCl (Copper(I) chloride). Instead of CuCl, you can use copper powder with HCl. In this reaction, Cu+ ion behave as a catalyst.
- Bromobenzene is formed when benzenediazonium chloride is treated with CuBr (Copper(I) bromide). Instead of CuBr, you can use copper powder with HBr. In this reaction, Cu+ ion behaves as a catalyst.
- When benzenediazonium chloride is treated with CuBr (Copper(I) bromide), Benzonitrile is produced.
- Iodobenzene can be produced by the reaction of benzenediazonium chloride and KI (potassium iodide). But, you should heat the reaction medium. For this reaction, Cu+ catalyst is not required as previous three reactions.
- You can prepare flurobenzene by the reaction of benzenediazonium chloride and HBF4 (fluoroboric acid).
 |
| Sandmeyer reactions of benzenediazonium chloride |
Join VKSU Help Desk ( On Telegram) – Click Here |
Preparing benzene by benzene diazonium chloride
This is just a single step reaction. Either you use H3PO2 or ethanol as the reagents with benzene diazonium chloride.
Benzene diazonium chloride and H3PO2 reaction
Benzene, acetaldehyde and nitrogen gas are given as products by this reaction. You can see, ethanol is oxidzed to acetaldehyde which is an aldehyde compound.
When benzene diazonium chloride reacts with sodium nitrite and copper (I), nitrobenzene is given.
Coupling reactions of benzene diazonium chloride
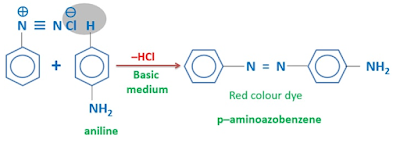
Benzene diazonium chloride reacts with phenol, β-napthol, aniline, 2-methylaniline to form azo dyes. In these reactions, a HCl molecule is eliminated.
Phenol and benzene diazonium chloride reaction
When benzene reacts with benzene diazonium chloride, red color organic dye is prepared. This dye is used to manufacture textiles. Reaction medium should be a basic.
β-naphthol and benzene diazonium chloride reaction
This reaction gives 1-(phenylazo)-2-naphthol, orange color dye. This dye also is used to prepare color textiles. Reaction medium should be a basic.
This reaction also give an organic dye. Benzene diazonium chloride reacts with aniline in basic medium to form p-aminoazobenzene which is Brownish-yellow needles with bluish cast.
When N-methylaniline reacts with benzene diazonium chloride in the presence of basic medium, p-methylaminoazobenzene is given.
 |
| N-methylaniline and benzene diazonium chloride reaction |
Join VKSU Help Desk ( On Telegram) – Click Here |
What is Diazotization Reaction?
Aromatic amine reacts with nitrous acid and mineral acid to form diazonium salt and produces water as a side product. This reaction is known as Diazotization Reaction. The reaction can be represented in words reaction form as follows –
Aromatic Amine + Nitrous Acid + Mineral Acid 🡪 Diazonium Salt + Water
Compounds in which amino or substituted amino group is bonded directly to an aromatic ring are known as aromatic amines. For example –
Nitrous acid is a weak and monobasic acid which is generally used in gaseous phase. Its formula is HNO2.
Nitrous Acid Structure :
Mineral acids are inorganic acids which give hydrogen ions when dissolved with water. Examples of mineral acids are HCl, H2SO4, HBF4 etc.
We can write diazotization reaction in the following form as well –
Example of Diazotization Reaction
Diazotization of Aniline
It is done by treating aniline with sodium nitrate and HCl at the temperature of 273K.
The Reaction Involved is Given Below :
 |
| Diazotization of Aniline |
Diazotization Reaction Mechanism
Diazotization mechanism can be explained in the following four steps –
Step 1. Formation of Nitrosonium Ion -
Nitrous acid reacts with mineral acid (mineral acid provides hydrogen ion) and forms nitrosonium ion. Reaction is given below-
Nitrous Acid + Hydrogen ion from mineral acid = Nitrosonium ion
Step 2. Formation of N-Nitrosamine -
In this step Nitrosonium ion reacts with aromatic amine to give N-nitrosamine. When nitrosonium ion reacts with aromatic amine, its positive charge shifts on the nitrogen of aromatic amine as nitrogen attached with aromatic amine gives its lone pair of electrons to nitrosonium ion. As a result of this, a nitrogen-nitrogen bond is formed between aromatic amine and nitrosonium ion. Now deprotonation takes place which gives N-nitrosamine as a product. Reaction involved is given below –
Aromatic Amine → Nitrosonium ion
Step 3. Formation of Diazohydroxide by Protonation and Deprotonation of N-Nitrosamine
Protonation of N-nitrosamine takes place followed by deprotonation of it. Which gives rise to diazohydroxide.
In this step protonation of diazohydroxide takes place which gives water and diazonium ion. Diazonium ion can be easily converted into diazonium salt.
Diazotization Titration
Diazotization titration involves diazotization reaction or formation of diazotization salt. In the diazotization titration process, we 1st weigh the sample and put it in the standard conical flask. Now conc. HCl and KBr are added in the flask and the rest of the volume is filled with the distilled water. This resulting solution is a standard solution. Now the appropriate quantity of standard solution is pipette out in another conical flask for titration. The temperature is maintained at 0-5℃. Now the solution is titrated with the NaNO2 solution until starch iodide paper turns blue. It indicates the end point.
Uses of Diazonium Compounds
Uses of Diazonium Compounds are as follows –
- It is used in the dye and pigment industry.
- It is used in document reproduction as these compounds are light sensitive and break down under UV or violet light.
- It is used in the synthesis of organic compounds.
- These compounds are used in explosive materials as solid diazonium halides are explosive in nature.
- It is used in fischer indole synthesis of triptan compounds and indomethacin.
- It is used in nanotechnology to exfoliate the nanotubes.
- It is used in the reaction called Meerwein Arylation which produces phenylated products.
Join VKSU Help Desk ( On Telegram) – Click Here |
















Lots of thanks for you and your team for providing all the answers and also all the information related to VKSU
ردحذفAgain thank you sir
By Rockstar Kush Maurya
إرسال تعليق